Commentary by Joan M. Chow, MPH, DrPH, Chief, Surveillance, Epidemiology, Assessment, and Evaluation Section, California Department of Public Health-Sexually Transmitted Disease Control Branch
Although over 1.4 million chlamydia cases were reported to the Centers for Disease Control and
Prevention (CDC) in 2013, there are potentially two to three times that number of infections as a result of asymptomatic infections.1-2 The development and implementation of evidence-based screening guidelines are key sexually transmitted disease (STD) program control strategies for closing that gap and further reducing the burden of preventable adverse reproductive health outcomes.
Although since 1993 the CDC and other professional medical organizations have developed chlamydia screening guidelines to efficiently target the highest risk populations, the guidelines developed by the United States Preventive Services Task Force (USPSTF) have been the standard by which chlamydia screening performance has been measured and monitored among providers.3 With the enactment of the Affordable Care Act (ACA), chlamydia screening is one of the key sexual health related measures included among covered preventive services to assure better patient care, population health, and reduce costs.4-5 Since nucleic acid amplification tests (NAATs) are the most sensitive and common diagnostic tests for chlamydia and are also bundled with tests for gonorrhea by design, changes in screening guidelines for chlamydia may potentially also impact gonorrhea detection. As with all USPSTF preventive service recommendations, the USPSTF periodically conducts a rigorous evaluation of published data demonstrating benefit and risk of chlamydia screening which was last conducted in 2007. We review here recent changes in the 2014 USPSTF recommendations for chlamydia screening and implications for clinical practice and chlamydia control in the population.
The recommendation level for chlamydia and gonorrhea screening was changed from an A to a B recommendation. To update the recommendation, USPSTF reconsidered all the evidence including those trials evaluated in the 2007 review as well as any that were published since.6-7 Only one randomized clinical trial, the Prevention of Pelvic Infection (POPI), has been published to evaluate the effect of chlamydia screening among young women since the last review.8
However, this randomized clinical trial did not have sufficient statistical power to detect a statistically significant small reduction in pelvic inflammatory disease (PID) incidence and reported no statistical significant effect of chlamydia screening. Thus, based on re-view of the POPI trial plus the older studies on the direct effects of screening, the USPSTF decided on a B recommendation, indicating that the net benefit for chlamydia screening is “moderate to substantial” rather than retaining the A recommendation reflect-ing “substantial” benefit.
Impact: There is likely limited impact on access to chlamydia screening since under the ACA goal of ensuring access to care, preventive services with either an A or B recommendation are available to at-risk persons without cost sharing. These services are available to individuals covered under non-grandfathered group or individual health plans, individuals covered under Medicaid expansion, and individuals covered through a plan chosen through a health insurance exchange. Additionally, there are financial incentives such as a 1% increase in the federal matching reimbursement rate if states choose to offer preventive services such as chlamydia screening.
However, this downgrade may diminish the perception of chlamydia screening as a priority preventive service if not mandated as in the case of grandfathered plans. Additionally, given the limited data available, it raises the question of whether the bar for evidence should be restricted to randomized control trials or should include observational studies, such as those that compare population-level screening with population trends in PID.9-10
Females 24 and under and pregnant females are still priority populations for chlamydia screening but the frequency of screening has been specified to “when a sexual risk assessment indicates new or persistent risk factors since the last negative test result.”
Previously, the 2007 recommendations stated that the optimal screening interval is not known but did note that the CDC recommended that women at increased risk should be screened at least annually. Although the latest USPSTF review still did not find any new evidence for the effectiveness of specific screening frequencies, the recommended screening interval was revised to be based on when risk factors changed. This could be considered broader in scope so as to reduce missed opportunities for chlamydia screen-ing among those whose risk has increased since last screening test. However, this revision raises the question of how providers can consistently interpret and implement this recommendation.
Impact: Effective implementation of this guideline may be achieved by performing regular sexual risk assessment. Efforts to increase current low rates of such risk assessment could be facilitated via clinical decision support enhancements to the medical record, but provider barriers to initiating discussions about sexual risk behaviors will continue to hinder further improvement in screening rates. Indeed, the lack of specificity regarding which risk behaviors are being monitored for change may actually result in lower screening rates.
Which risk behaviors would be important to consider for more frequent screening for young women as well as for primary screening of older women, i.e. age >24 years?
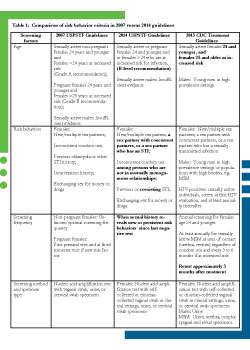
The 2014 guidelines are relatively consistent with the 2007 guidelines in identifying behavioral risk factors among females regardless of pregnancy status associated with chlamydial infection. Risk factors include new and multiple partners, incarceration history, exchange of sex for money or drugs, and seek-ing care in STD clinics. The 2014 guidelines continue to regard the evidence for screening asymptomatic males as insufficient, although encourage assessment and screening of young women who have a partner with concurrent partners was added in recognition of higher chlamydia transmission probability within partner networks. However, lack of specificity lingers in some such as previous (no time frame specified) or co-existing sexually transmitted infection (STI) (which STI not specified), whereas inconsistent condom use is now specific to those not in a mutually monogamous relationship. (See table 1 for comparison of risk behavior criteria in 2007 versus 2014 guidelines.)
Impact: The refinement of behavioral risk criteria for screening reflects trade-offs for efficient case finding. The change in screening frequency may result in decreased sensitivity and therefore missed opportunities to screen and treat females at risk. The lack of specific behavioral criteria may result in decreased specificity or over-screening as well as increased costs but with decreased return on investment.
There remains a need for studies to evaluate the efficiency of targeted chlamydia screening on the basis of behavioral risk factors. Such studies would need to use universal chlamydia screening and standardized behavioral data collection to compare the ability of different screening scenarios to detect the most infections while screening the least number of at-risk women.
Nucleic acid amplification tests (NAATs) are the most sensitive and specific tests to perform on specimens collected on FDA-cleared specimens: urine, cervical and vaginal specimens collect-ed in clinical settings.
Impact: The recommendation to use NAATs for the detection of chlamydia infections from genital and extragenital sites has been supported by the CDC 2014 Laboratory Guidelines as well as the Association of Public Health Laboratories based on multiple clinical test evaluations across clinical settings and populations.11 In this update however, the USPSTF criteria for recommending genital specimens remains limited to those that were part of the FDA clinical trials. Uptake of NAAT for chlamydia screening has been dramatic since their introduction to the point where the vast majority of specimens tested for chlamydia have been with NAATs. Use of any specimens not cleared by the FDA requires laboratories to con-duct verification studies to ensure similar performance of NAATs with home-collected vaginal, rectal, and pharyngeal swabs.
Differences with CDC and other national guidelines
Not all guidelines are created equal, and professional organizations have applied different standards by which the evidence sup-porting a guideline is weighed and can demonstrate that the benefits of screening outweigh harms. Un-fortunately inconsistency across guidelines translates to inconsistent application in clinical practice and missed opportunities for screening. For example, the recently released 2015 CDC STD Treatment Guidelines, which are also based on rigorous review of the evidence, retained the recommendation to conduct annual screening of females age 24 and younger as well as to conduct repeat screening of cases at 3 months, and test of cure for pregnant cases. Further, the CDC also recommended chlamydia screening of men having sex with men (MSM) if exposed at rectal and urethral sites and gonorrhea screening at all exposed sites.12
Chlamydial infections among MSM are associated with increased risk for HIV.13-14 As the vast majority of extragenital chlamydia and gonorrhea infections are asymptomatic, over half of asymptomatic extragenital chlamydia and gonorrhea infections would be missed if only urethral screening were conducted.15 Continued low reported rates for annual screening of young women and repeat screening, as well as low rates of screening among MSM being seen in HIV care and primary care settings reflect major clinical gaps for these priority populations.
It should be noted that the CDC guidelines also encompass a broader approach to chlamydia control strategies in that they also recommend concurrent treatment of partners as well as expedited partner therapy as effective tools for further interruption of ongoing transmission.
Conclusions
Current estimates of chlamydia screening rates from a variety of administrative and survey data sources consistently indicate that only about half of women seeking care at best are getting screened on an annual basis.16
However, the actual screening rates when based on self-report in population-based surveys tend to be even lower since not all will seek care, resulting in an even low-er uptake of screening and potential impact in the community. It is unknown whether these updated B-rated screening guidelines enhance access to chlamydia screen-ing for the other half of women who are missed. The criteria for evidence used by the USPSTF effectively limits our ability to expand testing to other populations at risk of acquisition and transmission, such as males age less than 24, men having sex with men, and men living in high prevalence communities.
While the current recommendations will likely ensure that access is maintained via the preventive services provision of the ACA, the inconsistency with other national guidelines creates the perception that demonstrated benefits are limited to women. It is up to STD programs and community providers to recognize all of the possible evidence supporting the value of chlamydia screening and strike the best balance between the benefits and costs of screening to protect the health of individual patients and the public.
References
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2013. Retrieved from http://www.cdc.gov/std/stats13/surv2013-print.pdf. Accessed April 22, 2015.
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187-93.
- Centers for Disease Control and Prevention. Recommendations for the prevention and management of Chlamydia trachomatis Infections, 1993. MMWR 1993;42:RR-12.
- Patient Protection and Affordable Care Act of 2010. Pub. L. No. 114–148 (March 23, 2010), as amended through May 1, 2010. Retrieved from http://www.healthcare.gov/law/full/index.html
- US Department of Health and Human Services. Preventive services covered under the Affordable Care Act. Retrieved from http://www.hhs.gov/healthcare/facts/factsheets/2010/07/preventive-services list.html
- Meyers DS, Halvorson H, Luckhaupt S, et al. Screening for chlamydial infection: An evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;147(2):135-42.
- LeFevre ML. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014; 161(12):902-10.
- Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: The POPI (prevention of pelvic infection) trial. BMJ. 2010; 340:c1642.
- Scholes D, Satterwhite CL, Yu O, et al. Long-term trends in Chlamydia trachomatis infections and related out-comes in a U.S. managed care population. Sex Transm Dis. 2012; 39(2):81-8.
- Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: Insights from exploratory time-series analyses. Am J Prev Med. 2010; 38(6):652-7.
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chla-mydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. 2014 Mar 14;63(RR-02):1-19.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Treatment Guidelines, 2015. MMWR Recomm Rep. 2015 June 5;64 (3): 55-56.
- Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with in-creased risk of HIV seroconversion. J Acquir Immune Defic Syndr .2010;53:537-43.
- Pathela P, Braunstein SL, Blank S, et al. HIV incidence among men with and those without sexually trans-mitted rectal infections: Estimates from matching against an HIV case registry. Clin Infect Dis. 2013;57:1203-9.
- Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41(1):67-74.
- Hoover KW, Leichliter JS, Torrone EA, et al. Chlamydia screening among females aged 15-21 years–multiple data sources, United States, 1999-2010. MMWR Surveill Summ. 2014;63 Suppl 2:80-8.
